HOW DO SOLAR CELLS WORK
In the last twenty years, the contribution of solar power to the world has grown
significantly.
significantly.
Energy from the sun is the most abundant and freely available
energy on the earth. And to utilize this free energy we have to take help from one of the most abundant elements on earth. We have to convert sand into 99.99% pure silicon to use in solar cells. A complex purification process is needed to get silicon from sand
energy on the earth. And to utilize this free energy we have to take help from one of the most abundant elements on earth. We have to convert sand into 99.99% pure silicon to use in solar cells. A complex purification process is needed to get silicon from sand
(SAND + CARBON [20000c]> RAW SILICON |98% pure|)
Then the raw silicon is converted into a gaseous
silicon compound form. Then this is mixed with Hydrogen to get highly purified
polycrystalline silicon. This silicon is then reshaped and converted into very
thin pieces called wafers. The silicon wafer is the heart of a photoelectric
cell. If you analyse the structure of the silicon atoms you can observe that
the silicon atoms are bonded together.
silicon compound form. Then this is mixed with Hydrogen to get highly purified
polycrystalline silicon. This silicon is then reshaped and converted into very
thin pieces called wafers. The silicon wafer is the heart of a photoelectric
cell. If you analyse the structure of the silicon atoms you can observe that
the silicon atoms are bonded together.
That’s why the electrons in the silicon structure have no freedom of movement.
Assume that fast-raised atoms with 5 valence electrons are injected into
it. Here one electron is free to move. When the electron gets sufficient
energy, it will move freely. If a solar cell uses this type of material and
when the light reflects them, the electrons get photon energy and will be free
to move. However, this free movement is random. It does not create a current
through the load.
it. Here one electron is free to move. When the electron gets sufficient
energy, it will move freely. If a solar cell uses this type of material and
when the light reflects them, the electrons get photon energy and will be free
to move. However, this free movement is random. It does not create a current
through the load.
A driving force is needed to flow the electrons unidirectionally. We need a PN junction to produce a driving force. Similar to N-type doping if you
inject Boron with 3 valence electrons into pure silicon, there will be one hole
for one atom. This is called P-type doping. If these N-type and P-type doped
materials are joined together, some electrons from the N side will migrate to the P
region and fill the holes available there. A depletion region is
formed in this way. There are no free electrons or holes. As
a result of the transfer of electrons, the boundary on the N side experiences a
slightly positive charge(+), while the boundary on the P side acquires a negative
charge(-). An electric field definitely formed
between these charges. A necessary driving force is produced by this electric field. When
the light makes contact with the P-N junction, it actually strikes the N-region
of the photovoltaic (PV) cell and subsequently penetrates and reaches the
depletion region. The electric field that the depletion region drives the electrons and
holes out of the depletion region.
inject Boron with 3 valence electrons into pure silicon, there will be one hole
for one atom. This is called P-type doping. If these N-type and P-type doped
materials are joined together, some electrons from the N side will migrate to the P
region and fill the holes available there. A depletion region is
formed in this way. There are no free electrons or holes. As
a result of the transfer of electrons, the boundary on the N side experiences a
slightly positive charge(+), while the boundary on the P side acquires a negative
charge(-). An electric field definitely formed
between these charges. A necessary driving force is produced by this electric field. When
the light makes contact with the P-N junction, it actually strikes the N-region
of the photovoltaic (PV) cell and subsequently penetrates and reaches the
depletion region. The electric field that the depletion region drives the electrons and
holes out of the depletion region.
A potential difference will develop between the P region and the N region because the concentration of electrons becomes so high. As soon as we connect any load between these regions, electrons start flowing
through the load. After completing their path, the electrons will recombine with the holes in the P region. In this way, a solar cell continually gives direct
current.
through the load. After completing their path, the electrons will recombine with the holes in the P region. In this way, a solar cell continually gives direct
current.
In
a real-world solar cell, the uppermost layer consisting of N-type material is
extremely thin and contains high levels of doping, while the bottom layer consisting
of P-type material is thicker and contains lower levels of doping. This is to be done to increase the
performance of the cell. You should know that the thickness of the depletion
region is much higher here than in the previous. This results in a more current generation by the PV cell.
benefit is that the thin upper layer allows for increased transmission of light
energy to the depletion region.
a real-world solar cell, the uppermost layer consisting of N-type material is
extremely thin and contains high levels of doping, while the bottom layer consisting
of P-type material is thicker and contains lower levels of doping. This is to be done to increase the
performance of the cell. You should know that the thickness of the depletion
region is much higher here than in the previous. This results in a more current generation by the PV cell.
benefit is that the thin upper layer allows for increased transmission of light
energy to the depletion region.
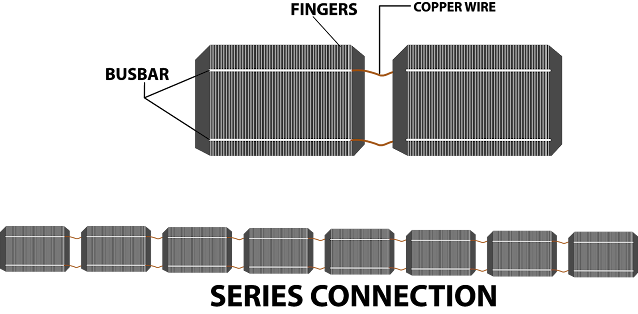
If we
analyse the solar panel then we can see that the panel has different layers. There
are different layers of a solar panel. The layer of cells is one of them. The electrons get collected to the busbars after the Photo Voltaic cells pass through the collectors. The top negative side of this cell is connected to the back of the next cell
through copper wire. It forms a series of connections. We get the solar panel when we connect these series-connected cells in parallel with other series-connected cells.
analyse the solar panel then we can see that the panel has different layers. There
are different layers of a solar panel. The layer of cells is one of them. The electrons get collected to the busbars after the Photo Voltaic cells pass through the collectors. The top negative side of this cell is connected to the back of the next cell
through copper wire. It forms a series of connections. We get the solar panel when we connect these series-connected cells in parallel with other series-connected cells.
A single Solar cell can produce only around 0.5 voltage. The combination of series and parallel connections of the cells
increases the current and voltage values to a usable range. To protect the Solar cells from shocks, vibration, humidity, dirt etc. there is an EVA shading layer on both sides of a solar panel.
increases the current and voltage values to a usable range. To protect the Solar cells from shocks, vibration, humidity, dirt etc. there is an EVA shading layer on both sides of a solar panel.
There are
two different kinds in the appearance of solar panels because of the different
internal crystalline latex structures. The two types are POLYCRYSTALLINE AND MONOCRYSTALINE. In the POLYCRYSTALLINE
solar panels, multi crystals are randomly oriented. If the chemical process
of silicon crystals is taken one step forward, the POLYCRYSTALLINE cells will be MONOCRYSTALINE.
MONOCRYSTALINE cells offer high electric conductivity. However, MONOCRYSTALINE cells are costlier and
that’s why it is not widely used.
two different kinds in the appearance of solar panels because of the different
internal crystalline latex structures. The two types are POLYCRYSTALLINE AND MONOCRYSTALINE. In the POLYCRYSTALLINE
solar panels, multi crystals are randomly oriented. If the chemical process
of silicon crystals is taken one step forward, the POLYCRYSTALLINE cells will be MONOCRYSTALINE.
MONOCRYSTALINE cells offer high electric conductivity. However, MONOCRYSTALINE cells are costlier and
that’s why it is not widely used.
That’s how a solar cell works.